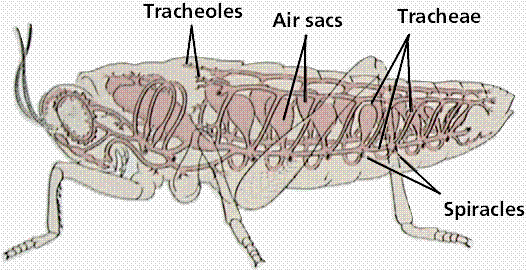
- Size And Surface Area -
Every organism's needs to be able to exchange substances or molecules with its environment in order to sustain life.
For example, Humans exchanges oxygen and carbon dioxide with its environment.
Cells need to take in oxygen (for aerobic respiration) and nutrients. // Also need to excrete (get rid of) waste products i.e. carbon dioxide and urea // heat needs to be exchanged so that the organism can maintain a constant temperature.
Smaller animals have a higher surface area : volume ratios
A mouse has a much larger surface area : volume ratio compared to an elephant.
However they are unable to perform exchange via their surface.
Although some mammals or other organisms have a large surface area to volume ratio, they are multi-cellular which means that they have a large diffusion distance and high demand as well as this they have a specialised exchange and transport system. They also have impermeable surface - this is to prevent pathogens entering and reducing water loss.
The exchange system would mean that the increases rate of diffusion of nutrients in and wastes out and the transport system had delivered nutrients and remove waste from all cells.
Animals with a high surface area : volume ratio tend to lose more water as it evapourates from their surface // small animals living in the desert would have kidney structure adapted to producing less urine to compensate for the loss of water.
High metabolic rates // small animals in the cold regions would have to eat large amount of high energy foods to maintain the amount of energy they use.
Large animals are adapted to extreme hot conditions // elephants have large flat ears to increase their surface area allowing them to lose more heat // hippos have behavioral adaptations to spend much of their day in water which helps them lose heat.

For example, Humans exchanges oxygen and carbon dioxide with its environment.
Cells need to take in oxygen (for aerobic respiration) and nutrients. // Also need to excrete (get rid of) waste products i.e. carbon dioxide and urea // heat needs to be exchanged so that the organism can maintain a constant temperature.
Smaller animals have a higher surface area : volume ratios
A mouse has a much larger surface area : volume ratio compared to an elephant.
However they are unable to perform exchange via their surface.
Although some mammals or other organisms have a large surface area to volume ratio, they are multi-cellular which means that they have a large diffusion distance and high demand as well as this they have a specialised exchange and transport system. They also have impermeable surface - this is to prevent pathogens entering and reducing water loss.
The exchange system would mean that the increases rate of diffusion of nutrients in and wastes out and the transport system had delivered nutrients and remove waste from all cells.
Animals with a high surface area : volume ratio tend to lose more water as it evapourates from their surface // small animals living in the desert would have kidney structure adapted to producing less urine to compensate for the loss of water.
High metabolic rates // small animals in the cold regions would have to eat large amount of high energy foods to maintain the amount of energy they use.
Large animals are adapted to extreme hot conditions // elephants have large flat ears to increase their surface area allowing them to lose more heat // hippos have behavioral adaptations to spend much of their day in water which helps them lose heat.