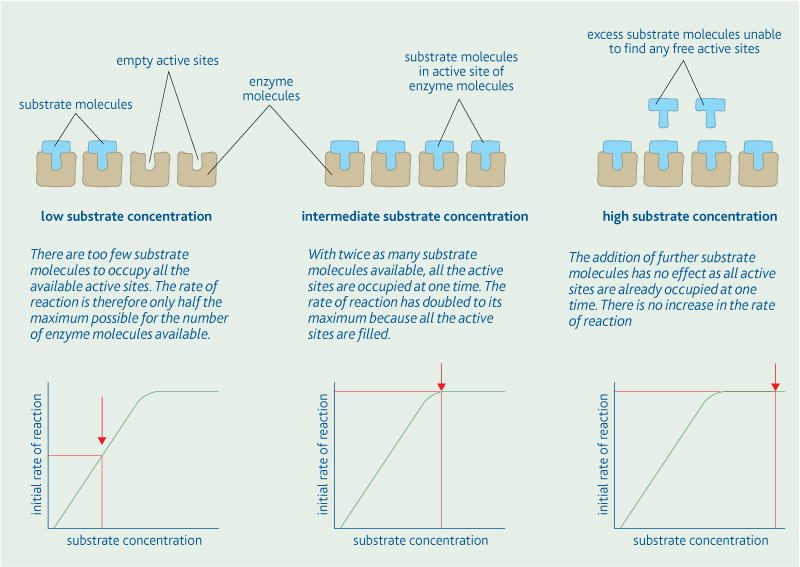
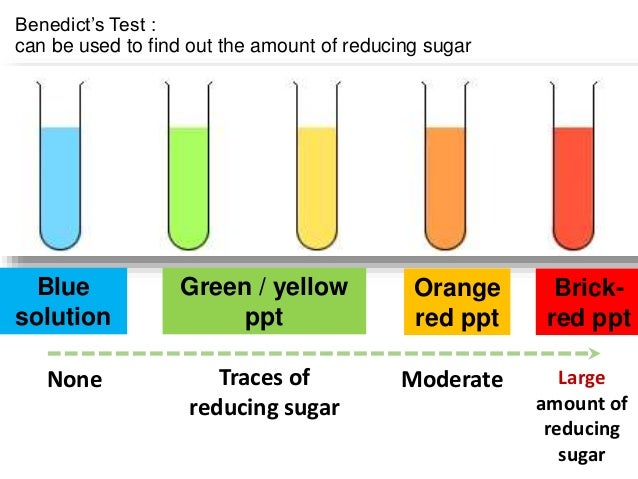
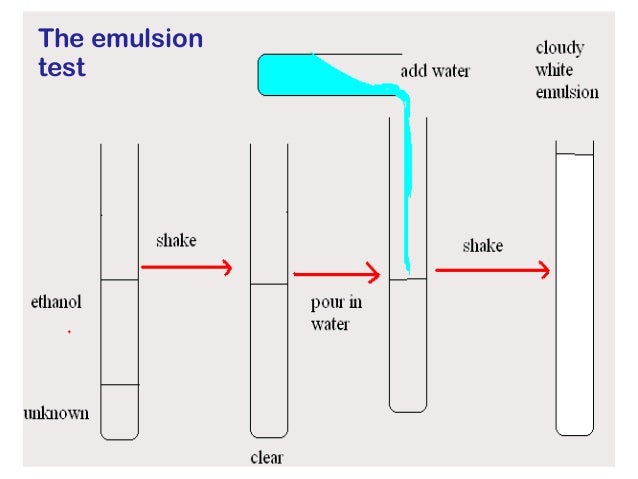
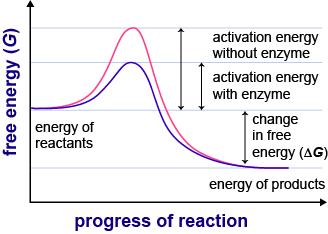
- Lipids -
- The Role Of Lipids -
S ● W ● I ● P
This helped me remembering the roles of lipids -
S ⇒ Source of energy
lipids provide twice the energy as carbohydrates at the same mass when hydrolysed
W ⇒ Waterproofing
Lipids are insoluble in water // plants and insects have a waxy cuticle reducing water loss whereas mammals have an oily secretion from sebaceous gland in the skin.
I ⇒ Insulation
Fats = slow conductor of heat // stored beneath the body surface to help retain heat
P ⇒ Protection
Fat stored around delicate organs i.e. Kidneys.
There are 2 types of lipids
Phospholipids and Triglyceride
So what is the difference?
Phospholipids have two parts
To remember there are two parts
PHOSPHOLIPIDS = PHO is repeated twice within the word - therefore two parts :)
Triglycerides have 3 fatty acids.
To remember there are 3 fatty acids.
TRIGLYCERIDE = TRI means 3
- Phospholipids -
- The two parts of the phospholipids is:
⇒ Hydrophilic 'head' = interacts with water (attracted to) // does not readily mix with fat.
⇒ Hydrophobic 'tail' = orients itself away from water (repels against) // readily mixes with fat.
- It has two ends and is said to be polar as they behave differently.
- Structure of phospholipids
⇒ Phospholipids are polar molecules - in aqueous environment phospholipid bilayer is formed hydrophobic barrier is formed between the cell (inside and outside).
⇒ Hyrdophilic 'head' helps hold at the surface of the cell-surface membrane.
⇒ Structure allows the formation of glycolipids // combining carbohydrates with the cell surface membrane. Acts as a cell recognition site.
- Triglycerides -
3 fatty acids joined to a glycerol
( TRI = 3 // GLYCERIDE = glycerol. )
The bond formed = ester bond (through condensation reaction)
There are 70 different types fatty acids, all have a carboxyl ( -COOH ) group attached with a hydrocarbon chain.
If this chain does not have carbon-carbon double bond = Saturated
If this chain has a single double bond = Mono-UnSaturated
If this chain has more than one double bond = Polyunsaturated
To remember the difference between unsaturated and saturated.
--------------------------------------------------------------------------------------------------------------------------
UN-SATURATED = There are two parts to the word which can imply double bond
SATURATED = A single word which can imply a single bond.
--------------------------------------------------------------------------------------------------------------------------
Structure of triglycerides -
⇒ High ratio of energy-storing carbon-hydrogen bonds to carbon atoms // excellent source of energy.
⇒ Low mass to energy ratio // good storage molecule = so much energy can be stored in small volume. Reduced mass in animals so able to move around.
⇒ Large, non polar & insoluble // storage does not effect osmosis in cells or the water potential
⇒ High ratio of hydrogen to oxygen atoms // release water when oxidised provides an important source of water.