- Protein -
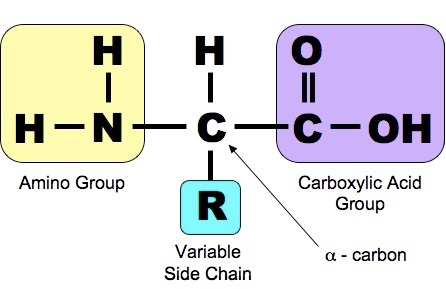
Structure of an amino acid
Amino acid is the back bone monomer units
Polymer ⇒ Polypeptide.
Polypeptides ⇒ proteins.
Every amino acid has a central carbon attached four different chemical groups
> Amino group -NH2 - which the amino part of the name of the amino acid is derived.
> Carboxyl group -COOH - acidic group gives the amino acid the acid part of the name.
> Hydrogen atom - H.
>R (side) group - variety of different chemical group 20 naturally occurring amino acids differ only in their R (side) group.
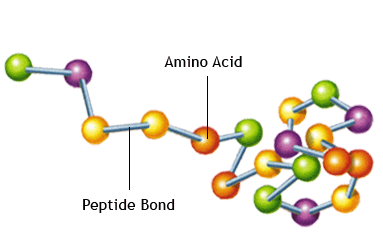
The formation of a peptide bond
A peptide bond formed through a condensation reaction as a result of joining two or more amino acids together.
The water is formed by combining an -OH from the carboxyl group of one of the amino acid with the -H of the other amino acid.
The amino acids are linked by a peptide bond between the carbon atom of one amino acid and the nitrogen atom of the other.
There are four structures of the protein molecule.
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
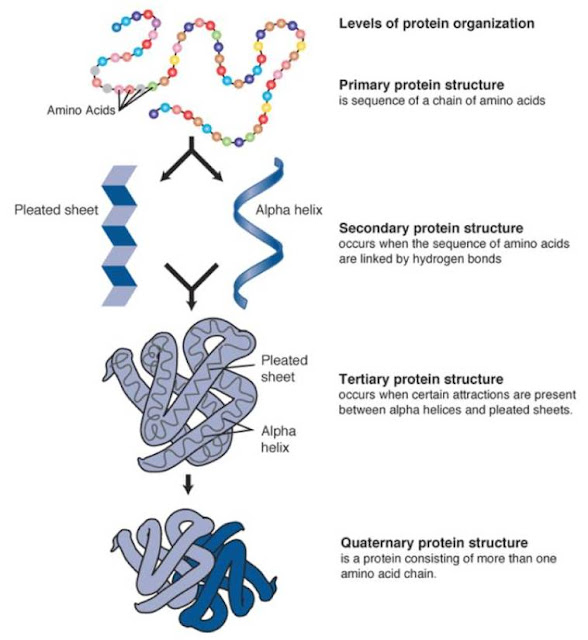
Primary structure
Polymerisation ⇒ This is a series of condensation reactions of many monomers of amino acids. As a result of this there is a long chain of amino acids (Polypeptide)
A sequence of amino acids in a polypeptide is the back bone formation of the primary structure of any protein, which is determined by DNA.
There are 20 naturally occurring amino acids joined in different sequences.
The primary structure determines the ultimate shape and function, a single change made to the amino acid sequence can lead to a change in shape of the protein and may stop its function completely.
Proteins have a specific shape which is specific to its function.
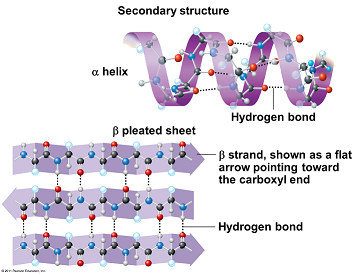
Secondary structure
The polypeptide chain is twisted into a 3-D shape ( coiled - α-helix )
This is due to the -NH and the -C=O the -NH has a hydrogen with a overall positive charge whereas the -C=O has an overall negative charge.
This readily forms weak hydrogen bonds.
Tertiary structure
The α helices of the secondary protein structure is twisted & folded to form an even more complex, 3-D and specific structure.
There are 3 bonds that maintains this Polypeptide helix structure.
- Disulfide bridges = fairly strong, not easily broken
- Ionic bond = formed between carboxyl and amino group // not involved in formation of peptide bonds. Not as strong as Disulfide bonds // Easily broken - by change in pH.
- Hydrogen bonds = many of them // easily broken
Quaternary structure
A number of individual polypeptides chains are linked together in various ways.
This structure, sometimes, includes non-protein // prosthetic// group i.e. iron containing haem group in haemoglobin.
--------------------------------------------------------------------------------------------------------------------------
There are two types of protein:
Fibrous protein and Globular protein
If you think of polypeptide as a string then you can understand that:
Fibrous protein is like collective pieces of string being twisted to for rope,
whereas,
Globular protein is like fewer pieces of string that is being rolled into a ball.
--------------------------------------------------------------------------------------------------------------------------



No comments:
Post a Comment