- Factors Affecting Enzyme Action -
There are many factors that effect enzyme action, this includes:
- Temperature.
- pH levels.
- Concentration.
However before delving into detail about how they effect the enzyme action. First, we have to see how enzyme-catalysed reactions are measured.
To measure the progress of an enzyme-catalysed reaction the time course is measured.
2 changes that are frequently measured:
> Formation of the products of the reaction.
> Disappearance of substrate.
Measuring rate of change
The gradient is equal to the gradient of the tangent to the curve at the point the tangent is the point at which a straight line touches the curve but without cutting across it.
You can draw the tangent to the curve at the point shown. Using this line you can find the gradient
a
= ㄧ
b
Effects of different factors on the rate of enzyme action it is important to stress the fundamental experimental technique of changing only a single variable in each experiment.
Rate of an enzyme reaction all the other variables must be kept constant when investigating the effects of a named variable.
Another thing to remember is that the active site and substrate is not the same as a lock and key.
Another thing to remember is that the active site and substrate is not the same as a lock and key.
Temperature ● pH ● Concentration
Factors effecting enzyme action.
Temperature:
When there a temperature increase, there is an increase in kinetic energy of molecule.
The molecules move around rapidly colliding with each other more often.
Enzyme-catalysed reaction = enzyme and substrates molecules come together more often in a given time.
More effective collisions = More enzyme-substrate complexes being formed.
At first the substrate does not fit as easily into the changed active site = slowing rate of reaction.
Human enzymes begin at temperature of 45℃.
Around 60℃ enzyme is so disrupted its stops working together = the enzyme denatures.
Denaturation is a permanent change and enzyme does not function again.
Many enzymes have an optimum temperature of around 40℃ in the human body.
Optimum temperature = the most/best favourable point or degree or condition at which an organism can work at which would obtain the best results.
pH:
pH of a solution is the measure of hydrogen ion concentration.
The pH of a solution is calculated using :
pH = -log₁₀[H⁺]
H⁺ concentration of 1 x 10⁻⁹ therefore has a pH of 9
An increase or decrease in pH reduces rate of enzyme action. If the pH level is extreme the enzyme becomes denatured.
The pH level affects enzymes works in two ways:
- pH alters the charge on the amino acids that make up the active site of the enzyme = substrates can no longer attach to the active site. In other words, enzyme-substrate complex cannot be formed.
- pH may cause the bonds maintaining the enzyme's tertiary structure to break = active site changes.
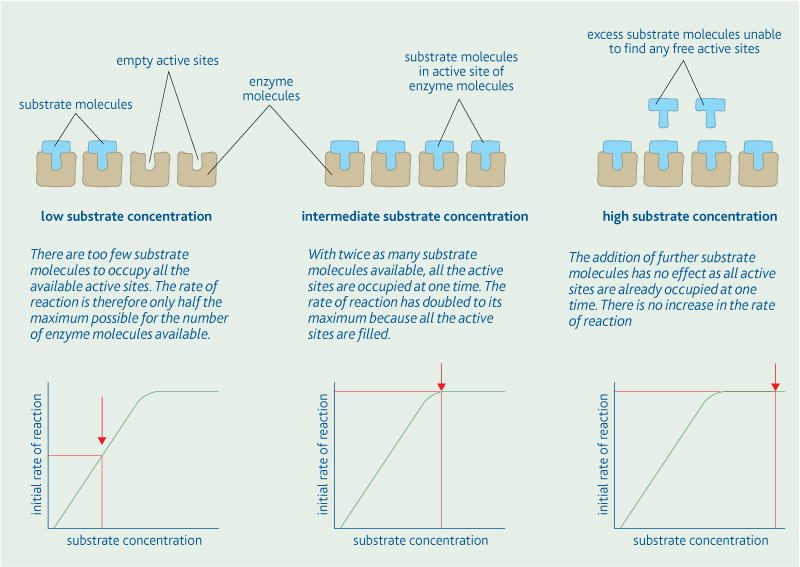
Concentration:
The active site can be reused to repeat the procedure on another substrate molecule once it is free. This means that enzymes are not used up during the chemical reactions, resulting in them working efficiently at very low concentrations.
- Low enzyme concentration: there are too few enzyme molecules to find an active site at one time.
- Intermediate enzymes concentration: all the substrate molecules can occupy an active site as the same time. The rate of reaction had doubled to it's maximum as a result.
- High enzyme concentration: adding more enzyme molecules has no effect as there are already enough active sites to accommodate all the substrate molecules.

No comments:
Post a Comment